-
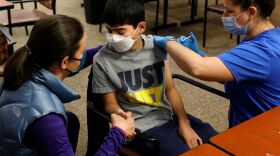
The Pfizer vaccine was approved for young kids aged 5 to 11 by the Food and Drug Administration.
-
Travis County officials say they are working with local school districts to begin vaccination efforts in the area.
-
The agency acted after an independent panel of scientists strongly supported the move. Kids could start getting vaccinated within the week.
-
After some debate, a group of scientists advising the FDA concluded that the vaccine's benefits outweigh its risks for young children.
-
Pfizer and BioNTech say that early trial results show their vaccine established a strong antibody response against the coronavirus. FDA review is still needed.
-
Ages 12 and older are now eligible to be vaccinated against COVID-19, the FDA and the CDC say. But when and where, and what about younger kids? You have questions. We have answers.
-
Pfizer said in late March that clinical trials found "100% efficacy and robust antibody responses" to the coronavirus in 12- to 15-year-olds.
-
The company said in late March that clinical trials showed the vaccine elicits "100% efficacy and robust antibody responses" in adolescents.
-
From Texas Standard: It all started with a battle over information: In one corner was the Texas Health and Human Services Commission. In the other were...
-
The deal will create the world's largest pharmaceutical company by sales, bringing together the makers of Viagra and Botox.
Play Live Radio
Next Up:
0:00
0:00
Available On Air Stations